Like all other disciplines, the healthcare industry is overwhelmed with the explosion of data and the ever-growing need to deliver insights. Increased focus on pay for performance, patient-centric care, and the limitations of Randomized Clinical Trials (RCT) to predict the real world performance of drugs are driving this industry to harness observational data to provide compelling arguments for drug effectiveness. However, the unique nature of such data poses its own challenges. First, data is available in diverse forms including patients’ narrations, electronic health records, adverse event data, and claims records. Secondly, different terminologies are used to describe the concepts. In order to process this multi-modal data, traditional approaches are not adequate. This paper explores an approach to effectively manage and integrate clinical data to assess real world performance.
A day in the life of a patient
Jane, a middle-aged law enforcement officer, visits a clinic complaining of a headache. The physician prescribes appropriate medication after basic health assessments. Jane then visits a different physician to report symptoms of urinary incontinence. Pelvic exercises to strengthen the bladder are recommended. While the patient’s symptoms have been efficiently treated, the two incidents put together tell a story that places Jane in considerable danger. These symptoms, in alignment with other conditions, could indicate Jane might be suffering from diabetes. This scenario highlights the risk of the prevalent approach to symptomatic treatment without accounting for the ‘whole’ health of patients.
If the physicians could have looked at Jane’s historical records of symptoms, diagnosis, prescriptions and the results of tests conducted across hospitals, they would have arrived at the conclusion that Jane needed treatment for Type 2 Diabetes. This is where technology can help improve patient care with a more holistic approach for consistent outcomes.
Real world evidence
Real world evidence is the clinical evidence regarding the usage and potential benefits or risks of a medical product derived from analysis of the data relating to patient health status and/or the delivery of healthcare routinely collected from a variety of sources. Real world evidence data can also be used for designing more efficient clinical trials, understanding a drug's benefit/risk profile, helping market access teams in economic model building and value demonstration, and understanding the market for launch planning. Furthermore, it could be used to understand adverse events alerts and drug compounding effects to help pharma companies reduce product cycle time and investment.
In other words, data being captured can help pharma companies answer questions like which molecules treat patients of a certain disease better? What are its side-effects? Which combination of drugs could have a negative impact? Besides, after the drug enters the market, continuous real world evidence plays a critical role. As data keeps filtering in, pharma companies can change drug molecules, issue warnings or even withdraw the drug.
From a pharma company’s perspective
In order to understand the effectiveness of drugs for patients like Jane, pharma companies are looking at an integrated view of patients’ journeys across treatment regimens. With the help of comprehensive analysis of real world data, it would have been easier to understand Jane’s case better.
Another area of interest for pharma companies and regulators is drug safety. While clinical trials do help identify any side effects of a drug, it is hard to determine adverse side effects of concomitant drug usage for all possible combinations of drugs in such controlled trials. Additional channels of information such as adverse events and social listening for patients’ comments are useful. A Real World Evidence (RWE) platform would help collect and process all observational data, develop tools and technologies to help analyze and take interventional measures as soon as such events are detected.
Moreover, pharma companies may be drawn more strongly—and more immediately—to use the data to gain a competitive advantage. For example, patients may require a 21-day course of a certain drug, costing (say) 100 USD. A competitor may claim to provide treatment for the same condition at half the price, but RWE could show that the course of treatment with the competitor’s drug takes twice the time and has several side effects.
On the commercial side, the market access team could use the data to understand competitive intelligence and which type of drug would work better in a certain geographical location. Brand managers can deepen their understanding of customer sentiments and strengthen brand loyalty. On the research and development end, the medical affairs team could use real world data to find out more about the ways to meet regulatory requirements by linking clinical and scientific results to real world data. This is also invaluable to biostatisticians and scientists in optimizing their research.
From a payer’s perspective
The steep rise in drug costs coupled with finite healthcare budgets is a major challenge. New medicines—some for life-threatening diseases—cost tens of thousands of dollars a month. Many of these medicines prolong lives but in many cases, the data from conventional trials is ambiguous, and it is hard to determine if the medicines are providing real value relative to their costs. RWE about the drug’s effectiveness can justify this for both the pharma company and the patient. Integrated RWE and RCT models also help generate meaningful evidence of a drug’s risk/benefit and cost/benefit profile, which is in line with payers’ expectations.
Need of the hour
Fortunately, there is no dearth of data in the world of medicine and pharma, just waiting to be leveraged. Physicians create electronic medical and health records, noting symptoms and recording their diagnoses using natural language. There are cross-sectional and longitudinal databases, which increasingly offer prospective ‘add-ins’ such as on quality of life. The use of health registries is growing and data of registered patients treated at a particular center for a particular condition can be easily analyzed. The FDA has an adverse events database, coded using the Medical Dictionary for Regulatory Activities for drug and therapeutic products. Academic literature has information about special characteristics of different medications and drug-drug interactions. Such data has been in existence for quite some time now. What is really required is the ability to link this data together through a common vocabulary so that analysts can use it for actionable insights.
The solution is to translate records into a common language and tag ontologies for drugs (generic names, indications, contra-indications, formulations) and medical conditions (symptoms, diagnosis, treatments, outcomes) so that the right meaning is captured across disparate records. Real world data involves a lot of unstructured information like lab and imaging data, and this requires a specially designed platform to store and give actionable insights.
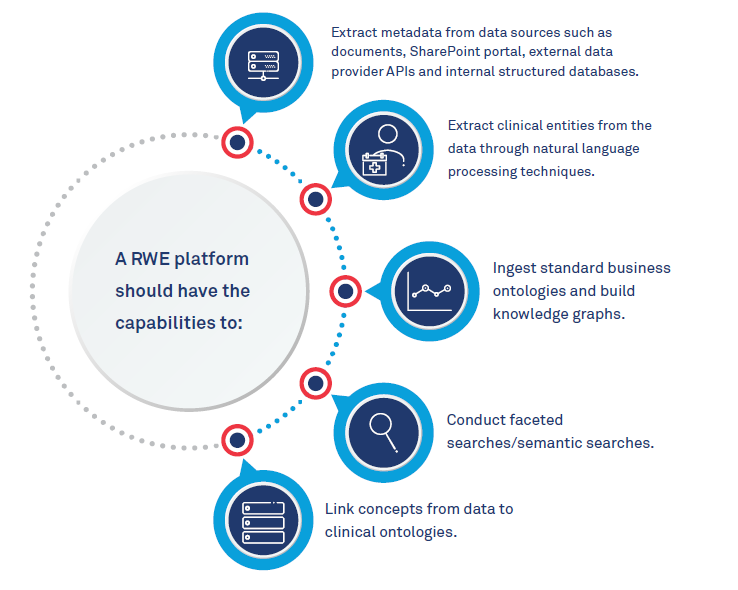
What it takes to create a RWE platform
The core component would be a powerful data platform to build and host clinical ontologies and link data to them. Researchers will need to find ways to standardize collection of data from more than 1 million volunteers from hospitals and clinics nationwide and find efficient ways to store large amounts of data. Privacy and the confidentiality of patients’ health information is another challenge. There is also a need to support ingestion of a variety of data types like documents, images, electronic medical records and other observational data from various sources like care providers, patients and third-party data providers. The ability to extract information from these data sources and link it to the clinical vocabulary is key.
Real World Evidence platform capabilities:
We need a cloud agnostic platform that enables customers to focus on their drug efficacy and drug safety study rather than managing and maintaining a complex data platform.
“As the breadth and reliability of RWE increases, so do the opportunities for FDA to also make use of this information.” —Scott Gottlieb, M.D., Commissioner, FDA
Beverly Chen
Principal Consultant, Wipro Ltd
Beverly is a thought leader in real world evidence. As part of the Health Business Unit strategy group, she is helping clients transform their business through outcomes-based models. A recent graduate of Wipro’s Global 100 leadership program, she has worked across functions and business units globally with rotations in consulting, delivery, and sales.
She holds a Master of Science degree in Biomedical Sciences from UC San Diego and an MBA from INSEAD.
Vivek Wandile
Practice Head, Wipro Ltd
With 25 years of experience, Vivek is currently part of Cloud computing team supporting deep learning research. He also supports solution development team that trains large scale neural networks. His areas of interest includes large-scale distributed systems, GPU accelerated computing, performance monitoring, information retrieval, knowledge construction and Machine Learning application.